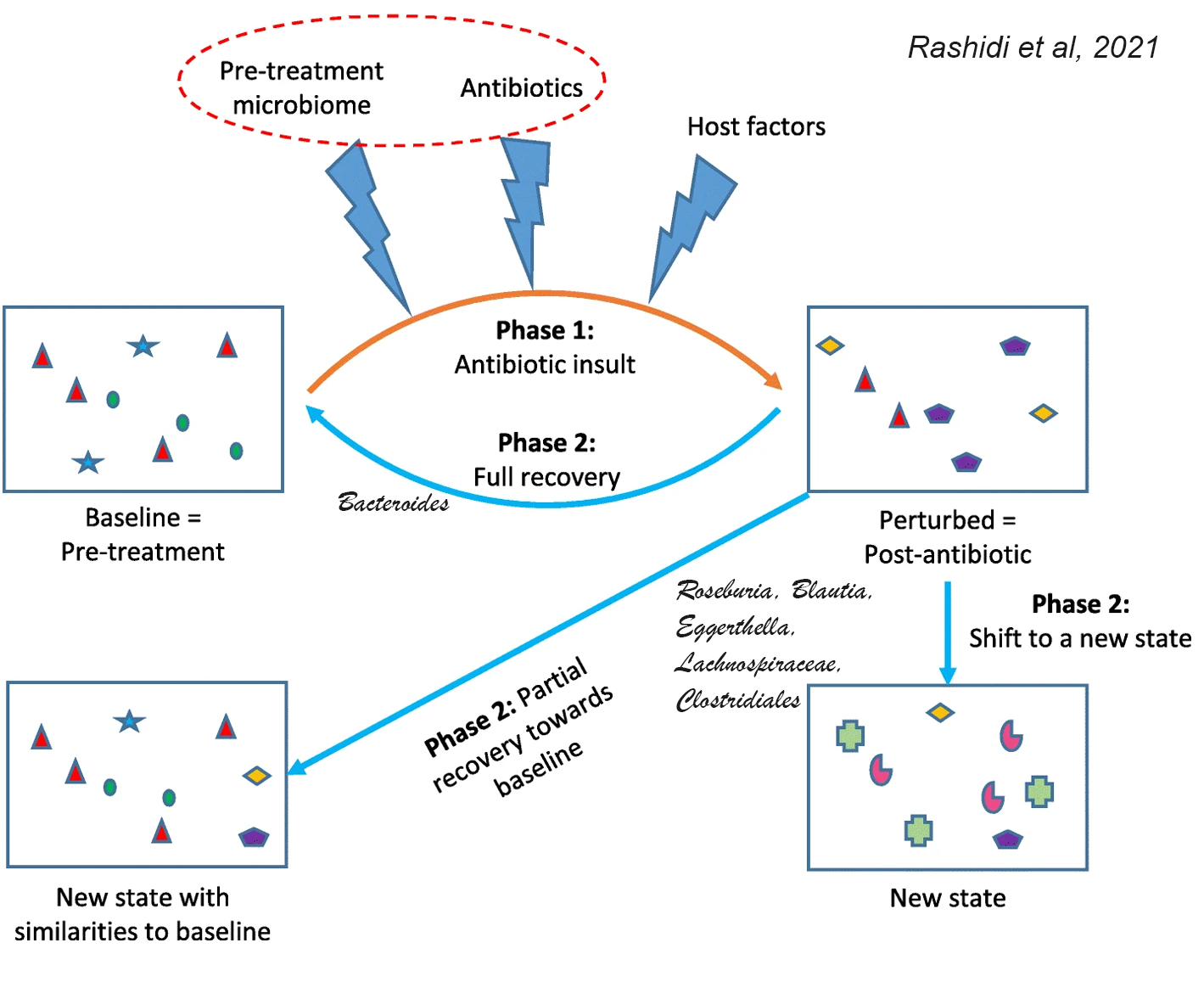
Antibiotics can effectively eliminate infection-causing bacteria, but they also perturb microbial communities in the body and this perturbation can be irreversible, depending on the individual. A new study demonstrates that the pre-treatment baseline gut microbiota is a major determinant of whether there will be complete or partial recovery,
or whether antibiotics will shift microbiome to completely new states with little resemblance to the baseline community. This is consistent with the role of pre-treatment microbiota in determining response to fecal microbiota transplantation (FMT) and dietary interventions.Principal component mixed effect regression using microbiota and granular antibiotic exposure data showed that microbiota departures from baseline depend on the composition of the pre-treatment microbiota. Penalized generalized estimating equations identified 6 taxa within pre-treatment microbiota that predicted the extent of antibiotic-induced perturbations.
In the final model, 5 baseline taxa (Roseburia, Blautia, Eggerthella, a Lachnospiraceae genus, and a Clostridiales genus) predicted larger microbiota departures from baseline, and one taxon (Bacteroides) predicted larger resistance to perturbations.
Next-generation precision antibiotics should be specific towards particular pathogens and their genes. They also should be tailored to the baseline host microbiome to prevent the development of functional gastrointestinal disorders.
REFERENCES
Rashidi, A., Ebadi, M., Rehman, T.U. et al. Gut microbiota response to antibiotics is personalized and depends on baseline microbiota. Microbiome 9, 211 (2021). https://doi.org/10.1186/s40168-021-01170-2