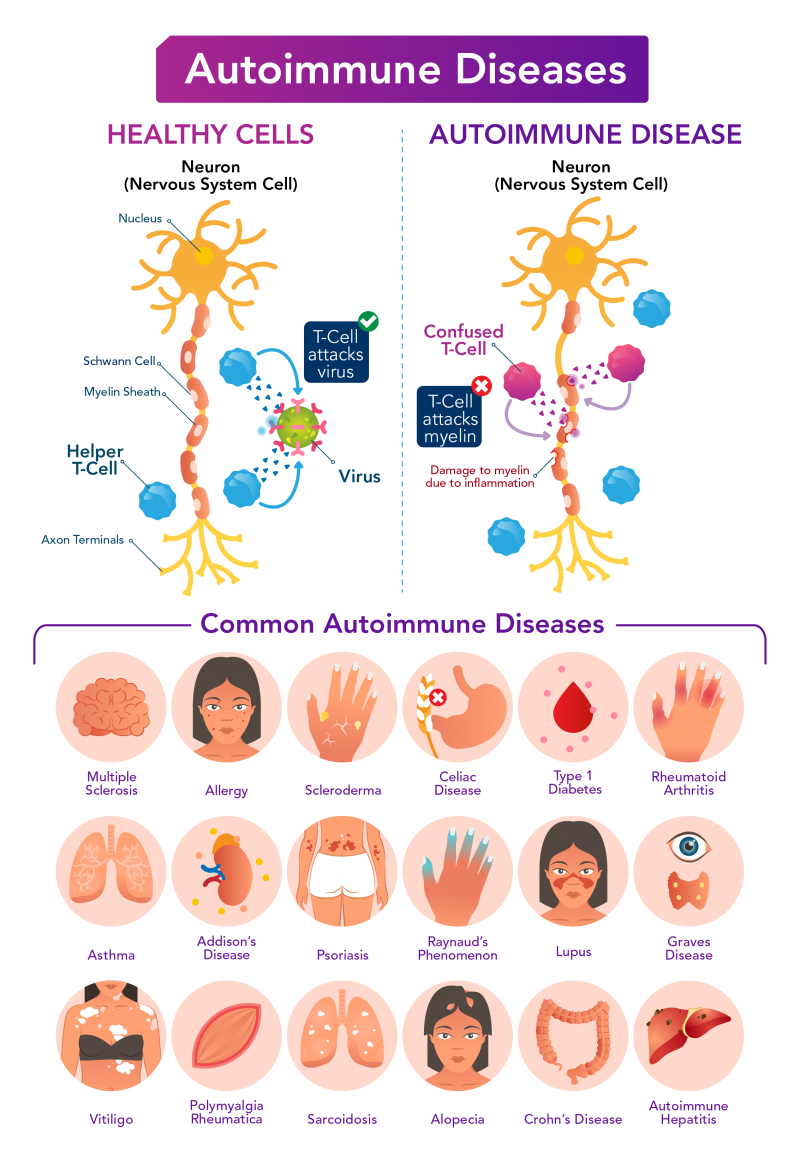
Autoimmune diseases occur when the immune system
attacks the healthy body tissue within digestive track, joints, vasculature
and other organ systems. This causes inflammation, pain,
diminished mobility, fatigue, and other non-specific
symptoms.
Nearly 4% of the world’s population and 5-8% of U.S. is affected by an autoimmune diseases, the most common of which include type 1 diabetes, multiple sclerosis, rheumatoid arthritis, lupus, Crohn’s disease, and psoriasis.
There is no evidence that any vaccines cause flares of autoimmune diseases, used to say doctors. However, there is limited data available since individuals with autoimmune diseases were excluded from phase I–III vaccine trials. And it is known that immunizations could cause flare ups (see, eg, this
study of 2020/2021 flu vaccines). Preliminary data from smaller studies and case reports after emergency-use-authorization for SARS-CoV-2 suggest there is a possibility.
A case of a white 55-year-old male who has been in sustained remission from rheumatoid arthritis for more than 2 years describes him developing an acute flare of his rheumatoid arthritis 12 h after the second BNT162b2 vaccination (similarly to
flares observed after COVID-19 infection). The patient was treated with intra-articular steroids with rapid improvement, and he is once again in clinical remission.
23-year-old woman who
developed acute reactive arthritis on her left knee joint after COVID-19 vaccination with Sinovac CoronaVac was back on her feet in 2 days, after she was administered a single intra-articular injection of 1 ml compound betamethasone.
A 20-year-old man with a history of multiple sclerosis experienced
acute myocarditis after the third dose of SARS-COV-2 vaccine (AstraZeneca vaccine). He had received the first and second dose of the SARS-COV-2 vaccine (Sinopharm
vaccine) 5 and 4 months before.
More recently published, 27 case reports from Israel, US and UK described
17 flares and 10 new onset immune-mediated diseases. 23/27 received the BNT - 162b2 vaccine, 2/27 the mRNA-1273 and 2/27 the ChAdOx1 vaccines. The mean age was 54.4 ± 19.2 years and 55% of cases were female.
A
study that compared 26 people with autoimmune disorders aged 24 to 89 (Rheumatoid arthritis, Crohn's disease, Psoriatic Arthritis, Sarcoidosis, Lupus, etc; none had been infected with SARS-CoV-2 prior to vaccination) with 42 healthy controls. Patients with autoimmune diseases had a marginal propensity towards more vaccine side effects compared with healthy controls: mild
fatigue and
myalgia were more frequent (53.8% vs 43.2% and 42.3% vs 31.6%) and so was
headache (38.5% vs 35.1%).
Fever, on the other hand, was completely absent in patients with inflammatory diseases while being reported by 13.5% of the healthy cohort. Arthralgia was comparable in both groups.
Researchers from two different rheumatology departments in Israel monitored
491 patients with autoimmune inflammatory rheumatic diseases (AIRD) and compared their reactions to 99 healthy controls. Shortly after receiving the vaccine, 1.2% of those with AIIRD (six patients total, age range: 36 to 61) developed their first case of
shingles compared to none of the controls.
Four of the six affected individuals had stable rheumatoid arthritis, one had Sjögren’s syndrome and another one had undifferentiated connective disease. Notably, one patient developed Herpes zoster despite being vaccinated for it two years prior to the reported event.
Multiple cases of apparent secondary immune thrombocytopenia (ITP), an unusual immune reaction triggered after SARS‐CoV‐2 vaccination have been reported and reached public attention.
One case was actually a
flareup for a patient with a past medical history of autoimmune bleeding disorder Immune thrombocytopenia (ITP). This patient received the first dose of SARS‐CoV‐2 mRNA‐1273 Moderna Covid‐19 vaccine 2 weeks prior to presentation.
Three other individuals that experienced
thrombocytopenia had known autoimmune conditions including hypothyroidism, Crohn's disease, or tested positive for anti‐thyroglobulin antibodies.
Given that a small percentage of patients with lupus and antiphospholipid syndrome have been previously shown to display serum
antibodies against PF-4 in association with thrombotic events constant vigilance is warranted.
REFERENCES
Buttari F, Bruno A, Dolcetti E, Azzolini F, Bellantonio P, Centonze D, Fantozzi R. COVID-19 vaccines in multiple sclerosis treated with cladribine or ocrelizumab. Multiple Sclerosis and Related Disorders. 2021 May 4:102983.
Geisen UM, Berner DK, Tran F, Sümbül M, Vullriede L, Ciripoi M, Reid HM, Schaffarzyk A, Longardt AC, Franzenburg J, Hoff P. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Annals of the Rheumatic Diseases. 2021 Mar 24.
Furer V, Zisman D, Kibari A, Rimar D, Paran Y, Elkayam O. Herpes zoster following BNT162b2 mRNA Covid-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford, England). 2021 Apr 12.
Lee EJ, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, Semple JW, Arnold DM, Godeau B, Lambert MP, Bussel JB. Thrombocytopenia following Pfizer and Moderna SARS‐CoV‐2 vaccination. American Journal of Hematology. 2021 Feb 19.
Moutsopoulos HM. A recommended paradigm for vaccination of rheumatic disease patients with the SARS-CoV-2 vaccine. Journal of Autoimmunity. 2021 May 1:102649.
Terracina KA, Tan FK. Flare of rheumatoid arthritis after COVID-19 vaccination. The Lancet. Rheumatology. 2021 Mar 30.
Toom S, Wolf B, Avula A, Peeke S, Becker K. Familial thrombocytopenia flare‐up following the first dose of mRNA‐1273 Covid‐19 vaccine. American Journal of Hematology. 2021 Feb 13.
Qi-jun An, De-an Qin & Jin-xian Pei (2021) Reactive arthritis after COVID-19 vaccination, Human Vaccines & Immunotherapeutics, DOI: 10.1080/21645515.2021.1920274
Watad A, De Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, Haddad A, Elias M, Zisman D, Naffaa ME, Brodavka M. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines. 2021 May;9(5):435.